
專長
細胞自噬研究、粒線體生物學、 老化生物學、細胞自噬誘導藥物、高通量藥物篩選
學歷
Ph.D., Department of Molecular and Integrative Physiology, University of Michigan, Ann Arbor, Michigan, U.S.A.
經歷
Post-doctoral researcher, Center for Autophagy Research, UT-Southwestern Medical Center, Dallas, Texas, U.S.A
辦公室
生物醫學大樓 五樓 R506 室
電話
886-2-2826-7127
Email
wchiang@nycu.edu.tw
個人網站
https://wchiang4.wixsite.com/chianglab
ORCiD
0000-0003-3149-435X
學經歷
2018 迄今
2013-2018
2012
國立陽明大學 生化暨分子生物研究所 助理教授
Center for Autophagy Research, UT-Southwestern Medical Center 美國西南醫學中心 細胞自噬研究中心 博士後研究員
Department of Molecular and Integrative Physiology, University of Michigan 美國密西根大學 生理學研究所博士
榮譽
2021
2020
2020
2019
2019
2014-2018
國立陽明大學醫學系優良教師琉璃獎座
國衛院CDG研究計畫
科技部優秀青年學者計畫
沈力揚教授基金會 講師級研究與進修獎助
國立陽明大學醫學院 學生網路教學評估優良教師
Centers of Excellence for Translational Research (CETR) Traineeship
指導學生獲獎
2025
2024
2023 2023 2022 2021 2021 2021 2020
2019
大專生 黃柏瑜獲科技部大專生計畫補助
大專生 吳佳諺獲年度健康科學文教基金會/國家衛生研究院「第 19 屆醫學系學生暑期研究計畫」補助
李沛靖 台灣粒線體學會年會 壁報競賽佳作獎
林怡辰 生物醫學年會 壁報競賽優勝
大專生 吳佳諺獲科技部大專生計畫補助
郭靖 台灣粒線體學會年會 壁報競賽第三名
劉沛函 台灣粒線體學會年會 壁報競賽佳作獎
朱瑋華 生化所碩士班優良論文評比 第三名
孫維澤 生物醫學年會 壁報競賽優勝
郭靖 台灣粒線體學會年會 優秀論文壁報獎
指導學生出國進修
2022
郭靖 美國羅徹斯特大學 (University of Rochester) 博士班
專利與技轉
專利名稱
發明人
技轉
Small molecule inducers of autophagy that function by disrupting Beclin 1/ Bcl-2 binding (細胞自噬誘發小分子藥物)
Jef DeBrabander, Qiren Liang, Beth Levine, Wei-Chung Chiang (姜為中)
US provision Filing No.:62680578 (Filing Date: 2018年6月4日)
Casma Therapeutics
專長— 選擇性自噬作用 (selective autophagy)、粒線體自噬(mitophagy)與相關疾病研究
研究方向
粒線體自噬作用 (Mitophagy)
粒線體是細胞的發電廠、產生能量的關鍵胞器,對多種細胞生理過程至關重要。粒線體功能異常會導致活性氧物質(ROS)增加,進一步引發發炎體(inflammasome)激活、基因毒性(genotoxicity)、腫瘤發生以及老化等一系列問題。因此維持正常的粒線體功能對於細胞存活與生物體的健康至關重要。
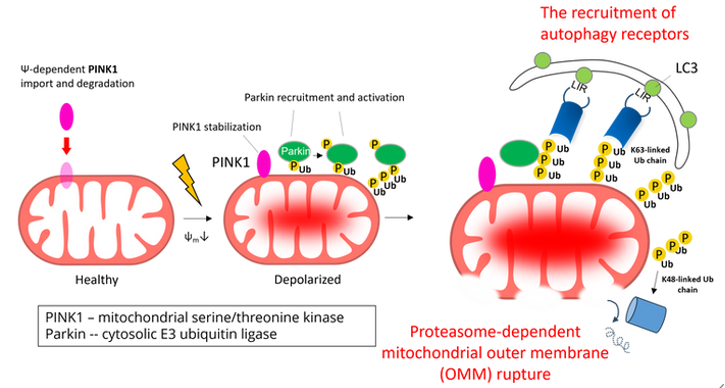
當細胞面對環境壓力(如養分缺乏、缺氧)等不利因素時,細胞能夠啟動許多促進環境適應的機制以確保存活。其中一種機制 — 自噬作用 (autophagy)為幫助細胞在不利環境下存活的重要反應。粒線體自噬(mitophagy)是一種特定形式的自噬作用,針對粒線體進行品質管控。通過此機制,真核細胞能夠選擇性清除多餘或損壞的粒線體來維持細胞健康。當粒線體損傷時,粒線體膜電位的下降會導致PINK1(一種絲氨酸/蘇氨酸激酶)在粒線體外膜(OMM)上的累積。穩定的PINK1會磷酸化與粒線體外膜蛋白上的泛素(ubiquitin),從而使Parkin(一種泛素E3連接酶)聚集到損壞的粒線體,Parkin在其上對多個外膜蛋白進行多泛素化(polyubiquitination)。最終,損壞的粒線體會被泛素結合的自噬受體(autophagy receptors)識別,並促進粒線體自噬作用的進行。
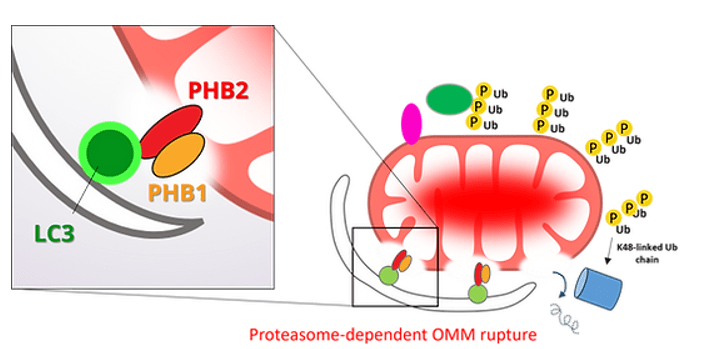
以往關於粒線體自噬機制的研究主要集中在粒線體外膜(OMM)上的事件,如PINK1、Parkin的活化、外膜蛋白的多泛素化以及自噬受體蛋白的招募。然而,觸發粒線體自噬的原因其實源自在粒線體內膜或基質中。因此,在粒線體內部應存在一些尚未發現的機制可以促進粒線體自噬作用。有趣的是,早期的研究已經發現,在粒線體自噬過程中會發生粒線體外膜破裂,這表明在自噬過程中,粒線體內膜可能會暴露,並被細胞質發生的自噬作用識別。為了驗證這一假設,我們採用了蛋白質體學方法,調查在粒線體自噬過程中,IMM蛋白是否會與LC3-II(一種自噬體上的膜蛋白)相互作用。我們發現了一種粒線體內膜蛋白 Prohibitin 2(PHB2),在粒線體去極化與外膜局部破裂過程中暴露於細胞質中,並透過一個典型的LC3相互作用區域(LIR)與LC3-II結合,藉此介導了損壞粒線體的辨識以及自噬清除。因此,我們的研究揭示了粒線體內膜的暴露本身可以作為一個促進粒線體自噬作用的機制。
研究方向一:粒線體自噬與老化、與其他生理功能(Mitophagy in longevity and healthiness)
適當去除損壞的粒線體對維持有機體的穩態至關重要。先前的研究表明,自噬缺陷與某些病理狀態相關,如老化相關疾病和神經退化性疾病(包括帕金森病和阿茲海默症),這些疾病的特徵之一是粒線體功能異常。觀察顯示,當粒線體自噬功能受損時,可能會導致以粒線體功能異常為特徵的神經退化性疾病的發展。因此,通過粒線體自噬降解去除不需要或損壞的粒線體,可能對維持有機體健康和預防年齡相關的病理變化具有重要作用。
粒線體自噬過去一直被認為是一種促進長壽的機制。先前在秀麗隱桿線蟲(C. elegans)中的研究表明,PDR-1(一種Parkin同源物)、PINK-1(一種PINK1同源物)的敲除會降低長壽突變株動物的壽命。這些研究表明Parkin、PINK1參與壽命調節。然而,我們無法得出明確結論是否這些蛋白的粒線體自噬功能是壽命調節所必需的。除了調控粒線體自噬,Parkin和PINK1還在其他細胞途徑中具有功能。因此難以準確區分PINK1和Parkin的粒線體自噬功能與其他粒線體功能對於老化所扮演的作用。總體而言,以一般遺傳學功能缺失(genetic loss-of-function)研究,目前無法直接證實粒線體自噬作為促進長壽機制的假設。
基於最近發現的粒線體內膜自噬受體 PHB2,我們利用點突變改變PHB2的LIR區域的氨基酸序列。該突變會阻斷粒線體自噬,但不影響PHB2的其他粒線體功能。因此,我們可以使用PHB2的LIR突變株將PHB2的粒線體自噬功能與其他PHB2的粒線體功能分開。使我們能夠採用獨特的遺傳學方法,更加精確地觀察粒線體自噬是否為一種促進長壽的機制,甚至研究粒線體自噬在其他代謝或生理功能的作用。我們目前在Phb2 LIR突變小鼠上發現了特定代謝與神經退化疾病的特徵,代表粒線體自噬的缺失會導致這些病理現象的發展。

研究方向二:粒線體外膜破裂的驅動力(Driving force of OMM rupture during mitophagy)
粒線體自噬過程中會發生粒線體外膜破裂,暴露出內膜的組成物,並被細胞質發生的自噬作用識別。這個機制對粒線體自噬來說是一個重要的過程。這件事的發生並不是單純地因為粒線體因損壞而瓦解,而是透過特定途徑調控的現象。目前已知粒線體外膜破裂需要Parkin以及蛋白酶體(Proteasome)的作用。但是具體外膜的是如何失去它的正常結構的? 我們對於於這方面的了解幾乎全無。
要解決這個問題,首先必須要有一個方便的工具可以有效測量粒線體外膜的破裂。在過往,蛋白酶保護測定法(Protease protection assay)與穿透式電子顯微鏡(TEM)常被用來探測膜破裂,但這些方法操作非常耗時且需要嚴格優化,限制了其高通量的應用性。為應對此挑戰,我們開發了一種偵測粒線體外膜破裂的生物感應器(biosensor),利用小分子誘導螢光蛋白感應分子的聚集來直接偵測外膜破裂位點。該方法可在粒線體外膜破裂處形成明顯的點狀訊號,並可在顯微鏡下快速的進行偵測、統計與分析。我們用這個新工具重新驗證了粒線體外膜破裂需要蛋白酶體和Parkin的活性,並採用較高通量的遺傳和藥理模式來研究粒線體外膜破裂的相關分子機制,揭示在細胞中的特定分子參與其中的外膜破裂過程。
研究方向三:膜通透性感應器的發展(Development of biosensor for membrane permeabilization)
在細胞內,膜的通透性改變對於細胞來說是一件大事,它可以是調控細胞功能的正常機制,但也可以代表危險或是死亡的訊號。因此有效的偵測膜的通透性改變可以幫助我們了解細胞穩態(cellular homeostasis)或是壓力反應(stress response)的調控機制。
目前我們仍在使用不同新技術的組合,針對上述膜破裂感應器進行更進一步的改良,以期能以更高敏感度在活細胞內即時偵測粒線體、或是其他胞器膜破裂的發生。長遠來說,未來這樣的工具將可以解決當今偵測胞器通透性的技術問題,更廣泛的運用在偵測與膜通透性相關的細胞生物學研究上。
Macroautophagy (hereafter referred to as autophagy) is a catabolic pathway by which cells sequester unwanted or damaged cellular proteins or organelles through a double membrane structure called the autophagosome. This process is mediated by a set of evolutionarily conserved genes, the autophagy-related (ATG) genes, which function in nucleation of the autophagosomal membrane, elongation of the autophagic membrane, sequestration of cytoplasmic constituents, and lysosomal degradation of the sequestered contents.
Selective autophagy is a homeostatic quality control process that targets specific cytoplasmic components to autophagosomes for lysosomal destruction. Diverse cargos have been identified as substrates for selective autophagy, including mitochondria (mitophagy), peroxisomes (pexophagy), endoplasmic reticulum (ERophagy), and viruses (virophagy). Mitophagy is the major pathway by which eukaryotic cells eliminate damaged or unwanted mitochondria as damaged mitochondria release reactive oxygen species (ROS), leading to inflammasome activation, genotoxic stress, promotion of tumorigenesis and aging. Defects in mitophagy are implicated in neurodegenerative diseases such as Parkinson’s disease, Alzheimer disease, and age-related pathologies. Thus, the proper removal of mitochondria is essential for organismal health in diverse eukaryotic species.
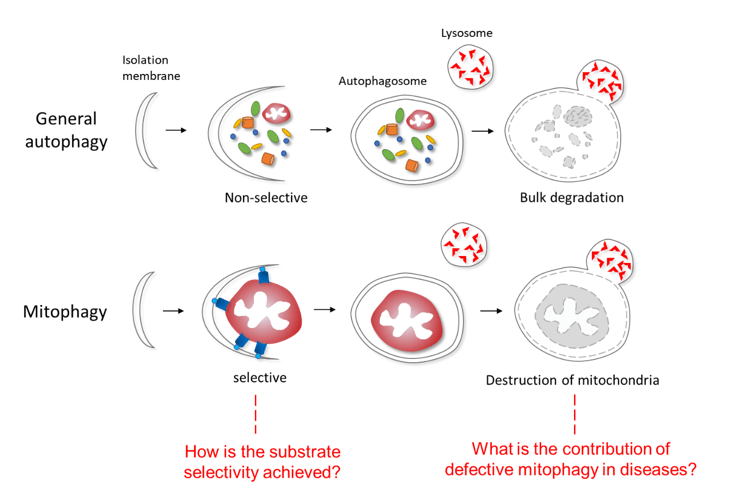
The primary research focus of our laboratory is to understand the molecular regulation and biological functions of mitophagy. A fundamental question in the autophagy field remains how cargos such as mitochondria are selectively targeted for autophagic degradation. We will use series of genetic, cell biology, biochemical approaches to understand the mechanisms that confer substrate selectivity in mitophagy and investigate the pathological consequences of mitophagy dysfunction in diseases using model organisms such as C. elegans and mice. These studies may guide future efforts in developing therapeutic options against age-related and neurodegenerative diseases and pave the way for the development of new treatments for patients with diseases associated with mitochondrial dysfunction.
研究著作
Wei-Hua Chu*, Yu-Shan Lin*, Jing Guo*, Wann-Neng Jane, Won-Jing Wang, Yu-Yang Lin, Pei-Han Liu, Po-Yu Huang, Wei-Chung Chiang. The ubiquitin-binding protein ANKRD13A mediates VCP-dependent mitochondrial outer membrane rupture during PINK1/Parkin-mediated mitophagy. J. Biol. Chem. DOI: 10.1016/j.jbc.2025.110739 (2025) (In press) (Featured article in “Editor’s Pick”)
Pei-Han Liu, Yu-Shan Lin, Wei-Hua Chu, Wei-Tze Sun, Po-Yu Huang, Jie-Rong Huang, Wei-Chung Chiang. Galectin-3 Directs Mitophagy in Response to Parkin-/Proteasome-Dependent Rupture of Mitochondrial Outer Membrane. Biol. Direct. (2025) (In press)
Xiaonan Dong, Qiren Liang, Yun-Zu Pan, Xiaoyu Wang, Yi-Chun Kuo, Wei-Chung Chiang, Xuewu Zhang, Noelle S. Williams, Josep Rizo, Beth Levine, and Jef K. De Brabander; Novel Bcl‑2 Inhibitors Selectively Disrupt the Autophagy-Specific Bcl-2−Beclin 1 Protein−Protein Interaction. ACS Med. Chem. Lett.,13: 1510−1516 (2022)
Jing Guo and Wei-Chung Chiang*. Mitophagy in Aging and Longevity. IUBMB Life, 74(4):296-316 (2022)
Álvaro F. Fernández, Salwa Sebti, Yongjie Wei, Zhongju Zou, Mingjun Shi, Kathryn McMillan, Congcong He, Tabitha Ting, Yang Liu, Wei-Chung Chiang, Denise Marciano, Gabriele Schiatarella, Govind Bhagat, Orson W. Moe, Ming-Chang Hu and Beth Levine. Disruption of the beclin 1-BCL2 autophagy regulatory complex promotes longevity in mice.Disruption of the beclin 1/Bcl-2 autophagy regulatory complex promotes longevity in mice. Nature. 558 (7708) 136-140 (2018).
Wei-Chung Chiang, Yongjie Wei, Yi-Chun Kuo, Shuguang Wei, Anwu Zhou, Zhongju Zou, Jenna Yehl, Matthew J. Ranaghan, Adam Skepner, Joshua A. Bittker, Jose R. Perez, Bruce A. Posner and Beth Levine. High Throughput Screens to Identify Autophagy Inducers that Function by Disrupting Beclin 1/Bcl-2 Binding. ACS Chem Biol. (2018). doi: 10.1021/acschembio.8b00421.
Yongjie Wei*, Wei-Chung Chiang*, Rhea Sumpter Jr., Prashant Mishra and Beth Levine. Prohibitin 2 is an Inner Mitochondrial Membrane Mitophagy Receptor. Cell. 168(1-2) 224-238 (2017). (*co-first author)
Wei-Chung Chiang, Tsui-Ting Ching, Hee-Chul Lee, Carol Mousigian and Ao-Llin Hsu. HSF-1 regulators DDL-1/2 link insulin-like signaling to heat-shock responses and modulation of longevity. Cell. 148(1) 322-334 (2012).
Wei-Chung Chiang, Daniel X. Tishkoff, Bo Yang, Joshua Wilson-Grady, Xiaokun Yu, Travis Mazer, Mark Eckersdorff, Steven P. Gygi, David B. Lombard, and Ao-Lin Hsu. C. elegans SIRT6/7 Homolog SIR-2.4 Promotes DAF-16 Relocalization and Function during Stress. PLoS Genetics, 8(9): e1002948.
Tsui-Ting Ching, Wei-Chung Chiang, Ching-Shih Chen and Ao-Lin Hsu. Celecoxib Extends Worm Lifespan Independent of COX-2 Inhibition. Aging Cell. 10(3):506-19 (2010).
Mu-Hwa Yang, Wei-Chung Chiang, Shyue-Yih Chang, Po-Min Chen and Kou-Juey Wu. Increased NBS1 expression as a prognostic marker of aggressive head and neck cancer and overexpression of NBS1 contributes to transformation. Clin. Cancer Res. 12, 507-515 (2006).
Yen-Chung Chen, Yi-Ning Su, Po-Chien Chou, Wei-Chung Chiang, Ming-Cheng Chang, Liang-Shun Wang, Shu-Chun Teng and Kou-Juey Wu. Overexpression of NBS1 contributes to transformation through the activation of phosphatidylinositol 3-kinase/Akt. J. Biol. Chem. 280, 32505-11 (2005).
Shih-Hung Yu, Wei-Chung Chiang, Hsiu-Ming Shih and Kou-Juey Wu. Stimulation of c-Rel transcriptional activity by PKA catalytic subunit beta. J. Mol. Med. 82(9):621-8 (2004).
