
專長
老化遺傳學、老化退化性疾病、飲食限制、 抗老化藥物開發、 生化及分子遺傳學
辦公室
生物醫學大樓 六樓 R613 室
電話
886-2-2826-7126
Email
alhsu@nycu.edu.tw
ORCiD
0000-0002-2864-3134
經歷
2023-24
2021
2018-21
2018-21
2018-21
2017-18
2013-
2013-18
2005-
2004-
2000-04
國立陽明交通大學 微生物暨免疫研究所 代理所長
國立陽明交通大學 生化暨分子生物研究所 特聘教授
中國醫藥大學 研究生事務長
中國醫藥大學 老化醫學研究中心 主任
中國醫藥大學 新藥開發所 教授
國立陽明大學 生化暨分子生物研究所 代理所長
國立陽明大學 生化暨分子生物研究所 教授
美國韋恩州立大學 生命科學系 兼任副教授
美國密西根大學醫學院 分子暨整合生理所 助理教授、終身副教授
美國密西根大學醫學院 內科老年醫學組 助理教授、終身副教授
美國加州大學舊金山分校 生物化學暨生物物理所 博士後研究員
榮譽
2024
2024
2019-23
2014, 18
2013-16
2008, 11, 13
2005
2001-04
中研學者獎
國科會傑出研究獎
國立陽明大學醫學院學生網路教學評估優良教師
科技部優秀年輕學者研究計畫 (三年)
科技部補助大專校院延攬特殊優秀人才
美國國家衛生研究院科研計畫補助
美國埃里森醫學基金會(Ellison Medical Foundation) 老化年輕學者獎
加拿大國家衛生院 博士後獎學金
指導學生獲獎
2023
2022
2022
2020
2020
2019
2018
2018
2017
2017
2017
2015
2015
2015
指導碩士生陳玉真獲國科會「補助研究生出席國際學術會議」出國參加 2023 24th International C. elegans Meeting
指導博士生王鳳雍參加生命科學院三分鐘生科論文英文口說競賽獲得特優獎
指導博士生王鳳雍獲科技部國際會議補助出國參加 2022 C.elegans MAPS Topic Meeting
指導碩士生蕭裕程所發表於Aging Cell之論文,獲選為Aging Cell 2020年度最佳論文第二名
碩班畢業生孫鈴媗獲得美國南加大/巴克老化研究所聯合學程全額獎學金攻讀博士學位
指導碩士生劉俞君獲教育部學海飛颺計畫補助至韓國中央大學交換學生
指導碩士生李奕萱獲科技部國際會議補助出國參加 2018 Asia Pacific C. elegans Meeting
碩班畢業生陳頡獲得美國UCLA全額獎學金攻讀博士學位
指導碩士生陳昭樺獲得陽明大學第六屆技術暨商業模式創意競賽第三名
指導碩士生孫鈴媗獲科技部國際會議補助出國參加 Keystone Symposia
指導碩士生孫鈴媗獲得生化所尹珣若論文比賽第三名
指導博士生Surojit Sural獲得20th International C. elegans Meeting壁報佳作獎
指導碩士生陳頡獲科技部國際會議補助出國參加20th International C. elegans Meeting
指導碩士生陳頡獲得生化所尹珣若論文比賽第三名
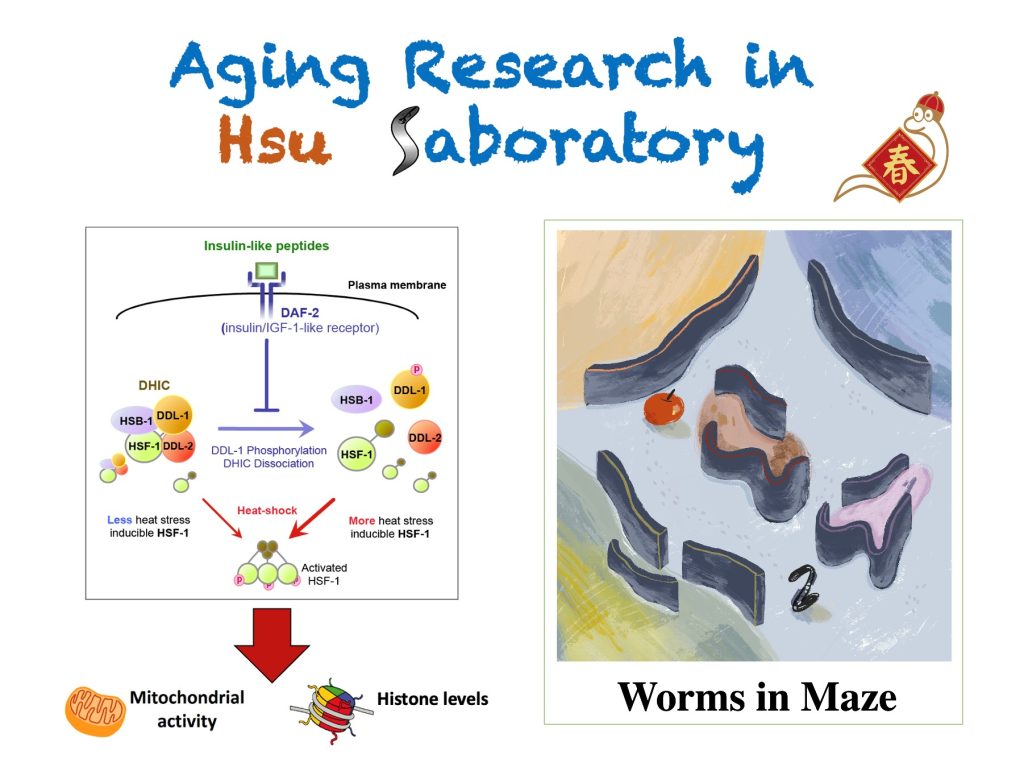
研究方向
Aging is a fundamental process characterized by progressive declines in physiological functions of multiple tissues and an increased likelihood of death. Accumulating evidence suggests that the rate of aging can be controlled by both hormonal and environmental cues. With its short lifespan, extensive genetics and well-described anatomy, the nematode C. elegans has been recognized as an excellent model system for aging studies. The main focus of our laboratory is to understand the biology of aging at the cellular and molecular level by investigating the genetic, environmental, and pharmacological factors that influence the rate of aging and longevity in model organisms. In particular, our current efforts are directed toward the following areas:
- Role of HSF-1 in longevity regulation : The importance of protein homeostasis and stress responses in the regulation of longevity and aging has been unveiled by recent studies. Heat- shock transcription factor (HSF-1) is the master regulator of the cellular defense mechanism against heat stress and has recently been directly linked to longevity. Our research has been focusing on (i) the cross-talks between HSF-1 and insulin/IGF-1 signaling, a well-studied longevity regulatory pathway, (ii) the upstream regulators of HSF-1, and (iii) the downstream targets of HSF-1 (e.g. HSPs, Histones) that plays important roles in aging regulation.
- Genes involved in the longevity response to dietary restriction : Dietary restriction (DR) or caloric restriction (CR) is known to result in a robust increase in lifespan while maintaining the physiology of much younger animals in a wide range of species. However, the molecular mechanisms underlying the longevity response of DR remain largely unknown. One of the main focuses of our laboratory is to study genes that mediate this longevity effect of DR. In particular, our current research has been focusing on genes involved in the TOR and AMPK pathway, RNA translation, and SAM-dependent methylation.
- Functional aging in the nervous system : As animals age, they exhibit a gradual loss in motor activity as well as cognitive functions. We aim to investigate the cellular and molecular origin of this age-associated motor activity decline by functionally characterizing the aging nervous system and muscles. We have also developed an innovative Worm Maze platform to assess the learning behavior in worms and to study the age-associated decline in cognitive functions.
- Whole-organism nascent transcriptomic profiling by single-nucleus RNA Seq : Using the most advanced snRNA-seq techniques, we aim to identify the temporal tissue-specific transcriptional changes in response to dietary or other environmental changes. We are also interested in identifying the inter-organ communication that drives the systematic responses.
- Pharmacological intervention that slows aging : The ultimate goal of our research is to develop new therapeutic strategies for combating aging and age-related diseases with the knowledge that we will obtain. We have been actively collaborating with several other groups to identify drugs or natural products that could slow the aging process or delay the onset of age-related neurodegenerative diseases, such as Huntington’s disease.
研究著作
Chen TY, Wang FY, Lee PJ, Hsu AL*, Ching TT*. (2024) Mitochondrial S-adenosylmethionine deficiency induces mitochondrial unfolded protein response and extends lifespan in Caenorhabditis elegans. Aging Cell, 23: e14103. (Epub Feb. 2024) doi:10.1111/acel.14103.
Oh KW, Kim DK, Hsu AL, Lee SJ. (2023) Distinct sets of lysosomal genes define synucleinopathy and tauopathy. BMB Reports, 56(12): 657–662.
Crombie1 E, Kim S, Adamson S, Dong H, Lu TC, Wu Y, Wu Y, Levy Y, Stimple N, Lam, Hey HW, Withers D, Hsu AL, Bay BH, Ochala J, Tsai SY. (2023) Activation of eIF4E- binding-protein-1 rescues mTORC1-induced sarcopenia by expanding lysosomal degradation capacity. J. Cachexia, Sarcopenia and Muscle. 14(1): 198-213. (Epub Nov 2022)
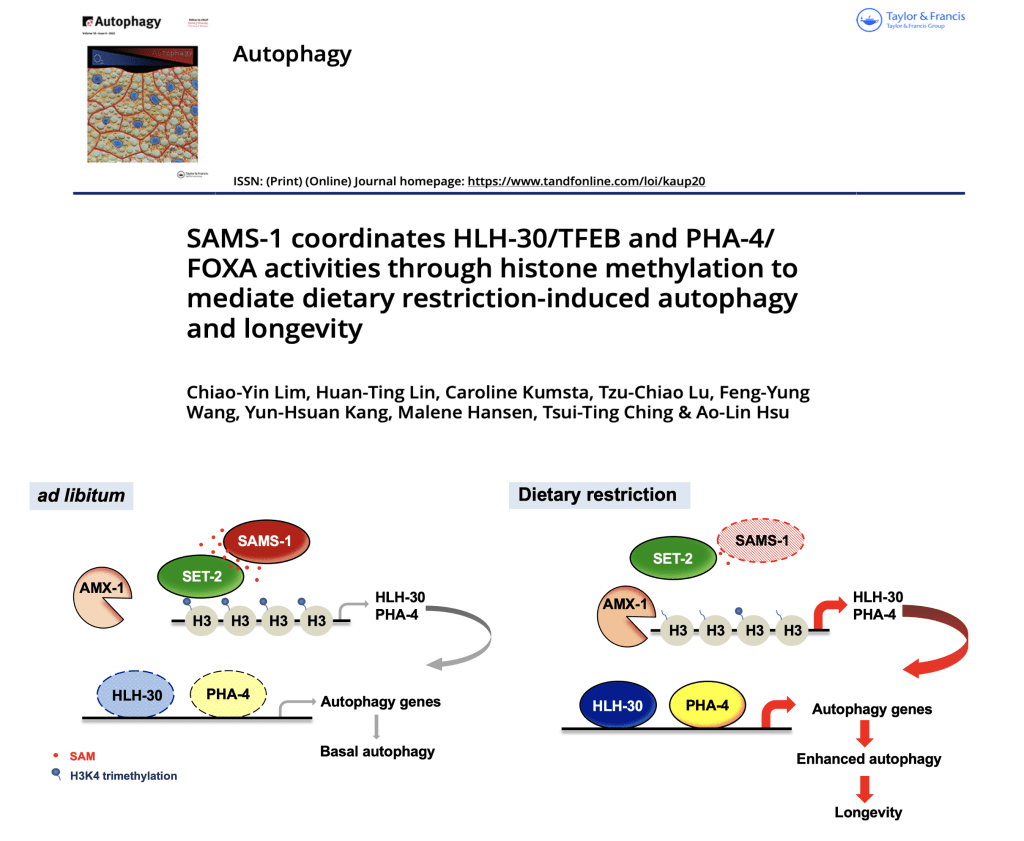
Lim CY, Lin HT, Kumsta C, Lu TC, Wang FY, Kang YH, Hansen M, Ching TT*, Hsu AL*. (2023) SAMS-1 coordinates HLH-30/TFEB and PHA-4/FOXA activities through histone methylation to mediate dietary restriction-induced autophagy and longevity. Autophagy. 19(1): 224-240. (Epub May 2022)
Wu KC, Chu PC, Cheng YJ, Li CI, Tian JK, Wu HY, Wu SH, Lai YC, Kao HH, Hsu AL, Lin HW, Lin CH. (2022) Development of a TCM-based agent for the treatment of cancer cachexia. J. Cachexia, Sarcopenia and Muscle. 13(4): 2073-2087.
Lin JL, Kuo WL, Huang YH, Jong TL, Hsu AL*, and Hsu WH*. (2021) Using convolutional neural network to measure the physiological age of C. elegans. IEEE/ACM Trans. Comput. Biol. Bioinform. 18(6): 2724-2732.
Gourgou E and Hsu AL*. (2021) A maze platform for the assessment of C. elegans behavior and learning. STAR Protocols. 2: 100829. doi.org/10.1016/j.xpro.2021.100829
Gourgou E, Adiga K, Goettemoeller A, Chen C, and Hsu AL*. (2021) C. elegans learning in a structured maze is a multisensory behavior. iScience. 24(4): 102284.
Chen CC, Lim CY, Lee PJ, Hsu AL, and Ching TT. (2020) S-adenosyl methionine synthetase SAMS-5 mediates dietary restriction-induced longevity in C. elegans. PLoS One. 15(11): e0241455.
Sural S, Liang CY, Wang FY, Ching TT* and Hsu AL*. (2020) HSB-1 signaling regulates longevity by elevating histone H4 levels to control mtDNA transcription. Science Advances,6(43): eaaz4452.
Lombard DB, Kohler W, Guo A, Gendron C, Han M, Ding W, Lyu Y, Ching TT, Wang FY, Chakraborty T, Nikolovska-Coleska Z, Duan Y, Girke T, Hsu AL, Pletcher S, and Miller R. (2020) High throughput small molecule screening reveals NRF2-dependent and -independent pathways of cellular stress resistance. Science Advances, 6(40): eaaz7628.
Ching TT, Chen YJ, Li G, Liu J, Xu XZS, and Hsu AL*. (2020) Short-term enhancement of motor neuron synaptic exocytosis during early aging extends lifespan in C. elegans. Experimental Biology and Medicine. 245(17): 1552-1559. doi:10.1177/1535370220950639.
Kuo CT, You GT, Jian YJ, Chen TS, Siao YC, Hsu AL, and Ching TT. (2020) AMPK-mediated formation of stress granules is required for dietary restriction-induced longevity in C. elegans. Aging Cell. 19(6): e13157.
Li ST, Zhao HQ, Zhang P, Liang CY, Zhang YP, Hsu AL, and Dong MQ. (2019) DAF-16 stabilizes the aging transcriptome and is activated in mid-aged C. elegans to cope with internal stress. Aging Cell. 18(3): e12896.
Sural S, Lu TC, Jung SA, and Hsu AL*. (2019) HSB-1 inhibition and HSF-1 overexpression triggers overlapping transcriptional changes to promote longevity in C. elegans. G3-Genes Genomes Genetics. 9(5): 1679-1692.
Li G, Gond JK, Liu J, Liu JZ, Li HH, Hsu AL, Liu J, and Xu XZS. (2019) Genetic and pharmacological interventions in the aging motor nervous system slow motor aging and extend lifespan in C. elegans. Science Advances. 5(1): eaau5041.
Son HG, Seo K, Seo M, Park S, Ham S, An SWA, Choi ES, Lee Y, Baek H, Kim E, Ryu Y, Ha CM, Hsu AL, Roh TY, Jang SK, and Lee SJ. (2018) Prefoldin 6 mediates longevity response from heat shock factor 1 to FOXO in C. elegans. Genes and Development. 32(23-24): 1562-1575.
Yuan Y, Hakimi P, Kao C, Kao A, Liu R, Janocha A, Boyd-Tesseler A, Hang X, Alhoraibi H, Slate E, Xia K, Cao P, Shue Q, Ching TT, Hsu AL, Erzurum SC, Dubyak GR, Berger NA, Hanson RW, and Feng Z. (2016) Reciprocal changes in phosphoenolpyruvate carboxykinase and pyruvate kinase with age are a determinant of aging in C. elegans. J. Biol. Chem. 291(3):1307-1319
Zhang B, Xiao R, Ronan EA, He Y, Hsu, A.L., Liu J, Xu XZS (2015) Environmental temperature differentially modulates C. elegans longevity through a thermosensitive channel. Cell Reports, 11(9):1414-142.
Huang CH, Hsu FY, Wu YH, Zhong L, Tseng MY, Kuo CJ, Hsu AL*, Liang SS*, Chiou SH* (2015) Analysis of lifespan-promoting effect of garlic extract by integrated metabolo-proteomics approach. J. Nutritional Biochem. 26(8) 808-817. (*co-corresponding authors)
Vukoti K, Yu X, Sheng Q, Saha S, Feng Z, Hsu AL*, and Miyagi M.* (2015) Monitoriing newly synthesized proteins over the adult life span of C. elegans. J. Proteome Res, 14(3): 1483-94.[Epub: Feb 25, 2015] (*co-corresponding authors)
Horikawa M, Sural S, Hsu AL, and Antebi A. (2015) Co-chaperone p23 regulates C. elegans lifespan in response to temperature. PLoS Genetics, 11(4):e1005023.
Kumsta C, Ching TT, Nichimura M, Davis A, Gelino S, Catan HH, Panowski SH, Baird N, Chu CC, Ong B, Yu X, Bodmer R, Hsu AL, Hansen M. (2014) Integrin-linked kinase regulates longevity and thermo-tolerance via neuronal control of HSF-1 in C. elegans. Aging Cell. 13(3): 419-430.
Liu J, Zhang B, Lei H, Feng Z, Liu J, Hsu AL, and Xu XZS. (2013) Functional aging in the nervous system contributes to age-dependent motor activity decline in C. elegans. Cell Metabolism. 18(3): 392-402.
Chiang WC, Tishkoff D, Yang B, Wilon-Grady J, Yu X, Mazer T, Eckersdorff M, Gygi S, Lombard DB*, and Hsu AL*. (2012) C. elegans SIRT6/7 homolog SIR-2.4 promotes DAF-16 localization and function during stress. PLoS Genetics. 8(9): e1002948.
Yuan Y, Kadiyala CS, Ching TT, Hakimi P, Saha S, Xu H, Yuan C, Mullangi V, Wang L, Fivenson E, Hanson RW, Ewing R, Hsu AL*, Miyagi M*, and Feng Z*. (2012) Enhanced energy metabolism contributes to the extended lifespan of caloric restricted C. elegans. J Biol Chem. 287(37): 31414-31426. (*co-corresponding authors)
Chiang WC, Ching TT, Lee HC, Mousigian C, and Hsu AL. (2012) HSF-1 regulators DDL-1/2 link insulin-like signaling to heat-shock response and modulation of longevity. Cell. 148(1-2): 322-334..
Ching TT, Chiang WC, Chen CS, and Hsu AL. (2011) Celecoxib extends C. elegans lifespan via inhibition of insulin-like signaling but not cyclooxygenase-2 activity. Aging Cell. 10(3): 506-519.
Ching TT and Hsu AL. (2011) Solid plate-based dietary restriction in Caenorhabditis elegans. J Vis Exp. (51): pii: 2701. [Epub: doi: 10.3791/2701].
Ching TT, Paal A, Mehta A, Zhong L, and Hsu AL. (2010) drr-2 encodes an eIF4H that acts downstream of TOR in diet-restriction-induced longevity of C. elegans. Aging Cell. 9(4): 545-557.
Hsu AL*, Feng Z, Hsieh MY, and Xu XZS*. (2009) Identification by machine vision of the rate of motor activity decline as a lifespan predictor in C. elegans. Neurobiology of Aging. 30(9): 1498-1503. (*co-corresponding authors)
Hansen M#, Hsu AL#, Dillin A, and Kenyon C. (2005) New genes tied to endocrine, metabolic and dietary regulation of lifespan from a Caenorhabditis elegans genomic RNAi screen. PLoS Genetics. 1(1): 119-134. (#co-first authors)
Hsu AL, Murphy C, and Kenyon C. (2003) Regulation of aging and age-related disease by DAF-16 and Heat-shock factor. Science. 300(5622): 1142-1145.
Dillin A, Hsu AL, Arantes-Oliveira N, Lehrer-Graiwer J, Hsin H, Fraser AG, Kamath RS, Ahringer J, and Kenyon C. (2002) Rates of behavior and aging specified by mitochondrial function during development. Science. 298(5602): 2398-401.
Garigan D, Hsu AL, Fraser AG, Kamath RS, Ahringer J, and Kenyon C. (2002) Genetic analysis of tissue aging in C. elegans: Heat-shock factor prevents progeria and proliferating bacteria kill the animal. Genetics. 161(3): 1101-1112.
Ching TT, Hsu AL, Johnson AJ, and Chen CS. (2001) Phosphoinositide 3-kinase facilitate antigen-stimulated Ca2+ influx in RBL-2H3 mast cells via a phosphatidylinositol 3,4,5-triphosphate-sensitive Ca2+ entry mechanism. J Biol Chem. 276(18): 14814-14820.
Hsu AL, Ching TT, Sen G, Wang DS, Bondada S, Authi KS, and Chen CS. (2000) A novel function of phosphoinositide 3-kinase in T-cell calcium signaling: A phosphatidylinositol 3,4,5-triphosphate-mediated Ca2+ entry mechanism. J Biol Chem. 275(21): 16242-16250.
Hsu AL, Ching TT, Wang DS, Song XQ, Rangnekar V, and Chen CS. (2000) The cyclooxygenase-2 inhibitor celecoxib induces apoptosis by blocking Akt activation in human prostate cancer cells independently of Bcl-2. J Biol Chem. 275(15): 11397-11403.
